Non-Hodgkin lymphoma trial tests chemotherapy plus antibody treatment
Indolent lymphomas are easy to treat, but hard to cure.
Indolent lymphomas are a type of non-Hodgkin lymphoma. Typically, they grow and move slowly – some people live 15 to 20 years with indolent lymphomas.
Chemotherapy can help some people with indolent lymphomas. But for tumors that come back, there's no standard treatment.
That's why oncologist R. Gregory Bociek, MD, is testing a new combination of three drugs. In this phase 1b clinical trial, the hope is to see partial remission without harmful side effects.
The trial combines three drugs:
- Copanlisib blocks several enzymes that increase cell growth. The clinical trial will test different doses of copanlisib.
- Nivolumab encourages a person's immune system's fighters – T cells – to kill cancer cells. It takes away a roadblock that prevents T cells from getting too trigger-happy in someone without cancer.
- Rituximab is an antibody that helps the body's immune system fight cancerous B cells.
"Combining antibodies with chemotherapy creates a synergistic effect larger than simply adding them together," explains Dr. Bociek. "The standard treatment for almost all lymphomas is to give chemotherapy and antibodies together." The trial's combination is not FDA-approved to treat this type of cancer and should be considered investigational.
The study is enrolling subjects at several cancer centers through the Big Ten Cancer Research Consortium. "The consortium helps my fellow Big Ten investigators around the country to collaborate, come up with ideas, and share results," says Dr. Bociek. "A study that takes 10 years at one location can take two or three years with everyone working together."
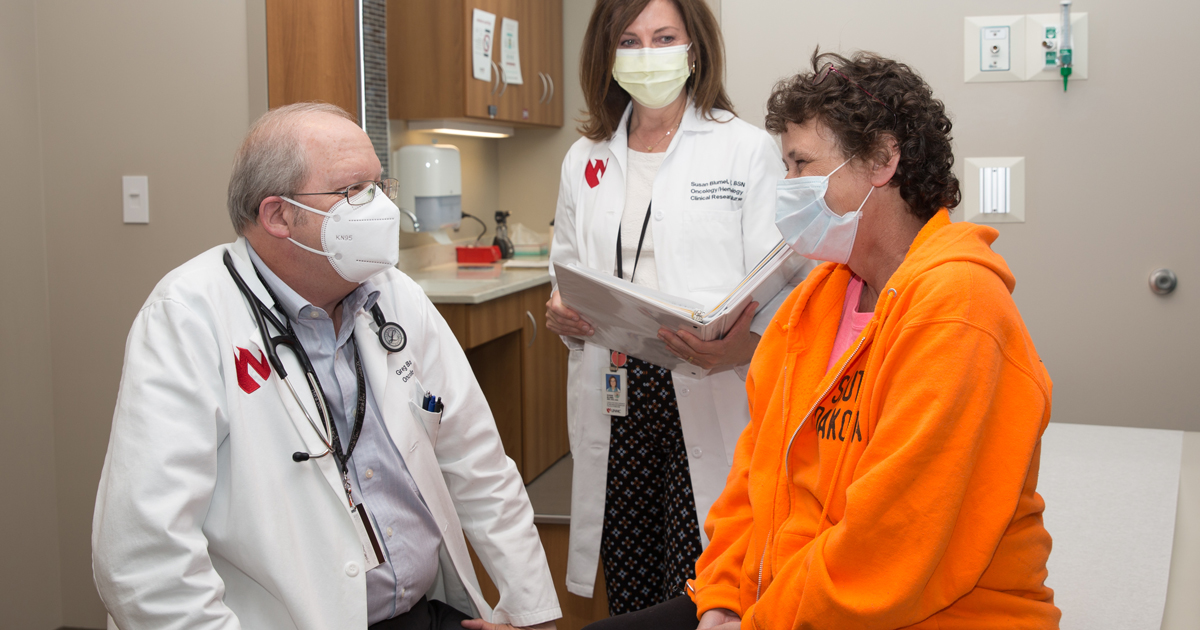
For patients like Dorothy Baucom, this trial is a godsend. Her cancer came back only eight months after chemotherapy. More aggressive chemotherapy and pills worked for a while, but her lymphoma kept coming back.
When Dr. Bociek told Baucom about the trial, she was excited and a little nervous. "Let's do this," she told him. After 2 months in the trial, she's doing well. "Dr. Bociek has been great to work with," she says. "I totally trust him."




